

Bentonite structure
While sharing a common elementary structure, the various types of bentonite are very different with regard to their chemical composition, as well as to the physical state of their constituents, which account for bentonite different properties and determine its various technological applications.
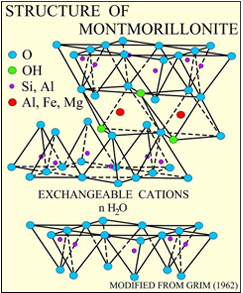
Montmorillonite is an aggregate of lamellar platelets, packed together by electrochemical forces and containing interposition water.
Each platelet consists of three sandwich-arranged layers: a central octahedral alumina (Al2O3) layer, and two tetrahedral silica (SiO2) layers. The silicon ion and the aluminium ion often undergo isomorphous substitutions by lower valence metals, such as magnesium and iron. In turn, these substitutions lead to a charge imbalance, compensated by exchangeable cations, in particular calcium (Ca2+), magnesium (Mg2+) and sodium (Na+) ions, together with water molecules bonded together by ion-dipole forces. These ions, with no more place inside the reticular structure, migrate to the external silica layers and are the main cause of hydration in the crystal lattice.
Therefore, each platelet can be assumed to have the following general formulation:
[ (Si Al)4 (Al Fe . Mg)2 O10 (OH)2 ]2 ∙ Mn ∙ mH2O
where the first member in brackets refers to isomorphic constitutions in the tetrahedral layers, the second member refers to isomorphic constitutions in the octahedral layer; and M and mH2O symbols refer to exchangeable cations and interposition water, respectively.
Every clay has a constant, maximum amount of exchangeable cations, as indicated by its cation exchange capacity (CEC), measured in milliequivalents per gram (meq/g) or, more frequently, per 100 grams (meq/100g). Bentonite CEC varies depending on the level of isomorphous substitutions occurred within the lattice. From a chemical point of view, bentonites can be distinguished depending on the quantity and quality of exchangeable bases: in particular, we have calcium bentonites and sodium bentonites, when the prevailing exchangeable cation is calcium or sodium, respectively. The largest deposits on earth contain calcium bentonites, which, however, have less hydration and swelling capacity than sodium bentonites.

